Abstract
High hyperdiploidy (HeH) is the most prevalent genetic subtype in paediatric acute lymphoblastic leukaemia (ALL) and is associated with a favourable outcome. HeH karyotypes have an increased modal number of 51-67 chromosomes and chromosome gain is non-random. Even though the outcome of paediatric patients with HeH is better than other subgroups, in adults it is far less favourable. The prevalence of the subgroup means that it accounts for a significant proportion of relapses; hence identifying risk factors within HeH is important. We recently developed a novel risk profile for HeH. Patients with both +17 and +18 together or patients with either +17 or +18 coupled with an absence of +5 or +20 defined a good risk (GR) group with a relapse risk <5% [Lancet Haem. 2021;8(11):e828-e39]. The remaining patients with HeH [poor risk (PR) group] had a relapse risk ~15% (i.e. similar to patients with intermediate risk genetics). In our original publication, we were restricted to using a single UK based clinical trial, UKALL2003, to validate our novel risk profile developed on data from ALL97/99. However, a comprehensive assessment of a novel risk profile requires validation on multiple independent cohorts but such cohorts are rarely readily available.
The HARMONY alliance (http://www.harmony-alliance.eu) is a European big data platform which has collected data on >115,000 patients with various haematological malignancies; including >10,000 patients with ALL. The aims of this project were to (a) determine if the UKALL-HeH risk profile can be validated in a large independent cohort comprising data from multiple clinical trials and from both childhood and adults patients; and (b) assess the added benefit of validating a risk profile using data from the HARMONY platform compared with a single a country-specific cohort.
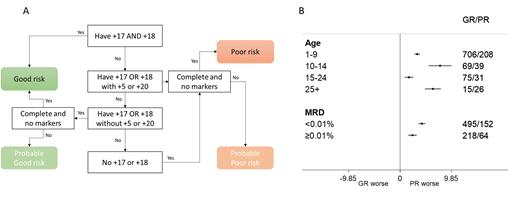
The ALL cohort within HARMONY comprised cases from multiple paediatric and adult ALL trials including UKALL2003. Only cases with karyotype written according to ISCN were eligible for this study. A search of the cohort revealed 1169 karyotypes with a modal number of 51-67 chromosomes. Karyotypes with t(9;22)(q34;q11) or chromosome patterns indicative of masked haploidy or low hypodiploidy were excluded. Using a custom built R function, we sorted karyotypes according to trisomic status of chromosomes 5, 17, 18 and 20 (fig1A). Karyotypes not assigned definitively to a risk group due to being incomplete or the presence of marker chromosomes were assigned to provisional groups but later combined with the definitive cases because their relapse rate was equivalent.
A total of 865 (74%) karyotypes had a UKALL HeH GR profile whilst 304 (26%) had a PR profile. The proportion of patients with a PR profile was significantly higher among those aged over 25 years compared to those under 25 (63% v 25%, p<0.001). Overall, patients with a PR profile had a significantly higher relapse rate at 5 years compared with those with a GR profile 6% (4-7) v 19% (14-23), log rank p<0.001. The hazard ratio from a univariate Cox model was 3.52 (SE 0.20 p<0.001). This effect was independent of MRD - hazard ratio for relapse from multivariate model was 3.30 (SE 0.26 p<0.001). The prognostic impact of the UKALL HeH profile was similar across all age groups examined including adults aged over 25 years but was greater among patients that were MRD negative (<0.01%) at the end of induction compared with MRD positive (≥0.01%) cases (fig1B).
Compared to using the UKALL2003 cohort alone for validation, HARMONY provided a validation cohort that was 60% larger with a broader age range: 1-24 vs. 1-70 years. In addition, the Cox model estimates were more accurate with smaller standard errors. The standard error for the hazard ratio for risk of relapse reduced from 1.11 to 0.20. Moreover, validation on cohorts of patients treated on protocols from other study groups provides additional evidence for the robustness of the risk profile.
In conclusion, we have validated the UKALL HeH risk profile on a larger and more heterogeneous cohort of patients. We confirm that the profile defines both a low risk subgroup of HeH paediatric patients that could be considered for treatment reduction, as well as a subgroup of HeH that has a risk of relapse similar to patients with intermediate risk genetics. In addition, we have demonstrated how the HARMONY data platform, which provides access to a standardised disease-specific datasets, can rapidly facilitate biomarker research.
Disclosures
Doubek:AstraZeneca: Consultancy, Honoraria, Research Funding; Janssen: Honoraria; AbbVie: Honoraria; AOP Orphan: Consultancy, Research Funding. Fielding:Amgen: Consultancy; Pfizer: Consultancy; Novartis: Consultancy. La Sala:Roche: Consultancy. Turki:CSL Behring: Consultancy; MSD: Speakers Bureau; Jazz Pharma: Speakers Bureau. Moorman:Amgen: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal